|
|||||||||

Comments
In
most circumstances, internal surfaces of stainless steel loops are inert
to all sample components. In the rare cases of adsorption, it can cause
poor precision of the adsorbed species. Here is what happens: When using
complete-filling (see Appendix F) some components
partition onto the loop surface. Upon injection, a mobile phase which
is stronger than the sample solvent desorbs these components, causing
the mass injected into the column to be larger than that caused by the
volumetric transfer. The mass adsorbed depends on the filling conditions:
strength of the sample solvent, the excess volume passed through the loop,
the loading flow rate, and the delay before injection.
This problem does not occur when loading less than 1/2 a loop volume; all sample is contained in the loop and there is no opportunity for concentration within the loop, regardless of the filling conditions. One remedy to the problem is to switch to the partial-filling method.
Adsorption sometimes accounts for a large difference in the precision of some peaks observed between two laboratories, when the filling conditions of the two labs are different. For example, although both groups may be injecting the same nominal volume, one group may be using partial-filling, and the other using complete-filling.
Symptom #8
When using the complete-filling method, the precision of some peaks is much poorer than that of other peaks (see Fig. 28).
The poor precision of problem peaks may be caused by their adsorption onto the internal surfaces of the sample loop or the rotor seal. This rare problem is more likely when:
- Using ion pairing reagents and not all sample components form ion pairs.
- The sample solvent is weaker than the mobile phase.
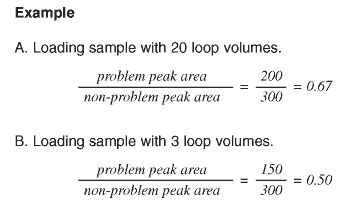
To test: Load sample using twenty loop volumes. Inject immediately. Determine the ratio of a problem peak area to a non-problem peak area. Repeat, using three loop volumes. This passes less total sample over the adsorbing surface, so less will be adsorbed during loading and subsequently desorbed during injection. Inject immediately. Determine the area ratio. Ignore the fact that all peaks in the second chromatogram may have slightly smaller areas than the corresponding peaks in the first chromatogram.
- If, in the second chromatogram, the ratio of problem peak area to non-problem peak area is smaller than the ratio in the first chromatogram (see Fig. 28), see the cause below.
- If the ratio is unchanged, adsorption is probably not occurring.
Fig. 28. In this example sample is adsorbing, since the ratio of problem peak to non-problem peak is smaller with the lower loading volume (three loop volumes) than with the higher loading volume.
Cause
The problem peaks are adsorbing.Solution
Use mobile phase as sample solvent. Load the same volume, using the same flow rate, and with the same time interval before injection (see comments for a detailed explanation).
© 2000 Rheodyne All rights reserved